According to foreign media reports, an interdisciplinary research group at the Technical University of Munich (TUM, Germany) has produced platinum nanoparticles for the catalytic process of fuel cells. The size of the new catalyst has been optimized and has been realized. Compared with the platinum particles made by the best industrial process, its performance has doubled. Fuel cells can replace batteries and become a source of power for electric vehicles. Fuel cells consume renewable hydrogen (such as the use of surplus electricity from wind power plants to produce hydrogen), but platinum used in fuel cells is very scarce and particularly expensive, limiting the large-scale application of fuel cells. A research team at the Technical University of Munich in Germany has now optimized the size of the platinum particles, which is twice as high as the particles produced by the current commercial process. The research team was led by Roland Fischer, a professor of inorganic and organometallic chemistry, Aliaksandr Bandarenka, Department of Physical Energy Exchange and Storage, and Alessio Gagliardi, a professor of energy conversion simulation for nanosystems. In fuel cells, hydrogen reacts with oxygen to produce water, which produces electricity in the process. To optimize such conversion, a catalyst is needed on the electrode, and platinum plays a central role in the oxygen reduction reaction. To find the ideal solution, the research team created a computer model of the entire system. The core question is: how small can the platinum cluster be, and at the same time, can it have a highly active catalytic function? Professor Fischer said: "It turns out that platinum particles have a certain optimal size." Platinum particles of about 1 nanometer and containing about 40 platinum atoms are ideal. Bandarenka said: "Although this size of platinum catalyst is small, it has a large number of high activity points." The interdisciplinary cooperation of the Catalyst Research Center (CRC) is an important factor for the research team to obtain research results. Combining modeling theory, collaborative discussions and physical chemistry knowledge obtained from experiments, the researchers finally made a model that showed How to design the catalyst according to the ideal shape, size and size distribution of components in the fuel cell. In addition, CRC has the expertise to create and experimentally test such platinum nanocatalysts. Twice the effect of the best catalyst The experiment confirmed the researchers' theoretical predictions. Garlyyev said: "The catalyst we produced is twice as effective as the best catalyst on the market." However, because the current platinum content has only been reduced by 50%, it is still not enough to achieve commercial application. It must be reduced by 80%. In addition to such spherical platinum nanoparticles, the researchers also hope to study platinum nanoparticles with more complex shapes but higher catalytic activity. The computer model is ideal for modeling, but andarenka said: "The more complex synthesis of more complex shapes required." (Author: Yuqiu Yun) Pellin Broca Prism,Optical Pellin Broca Prism,Fused Silica Broca Prism,Pellin Broca Silica Prism Lambda Research Optics ChangChun,LTD. , https://www.lambdachina.com
(Source: Technical University of Munich, Germany) 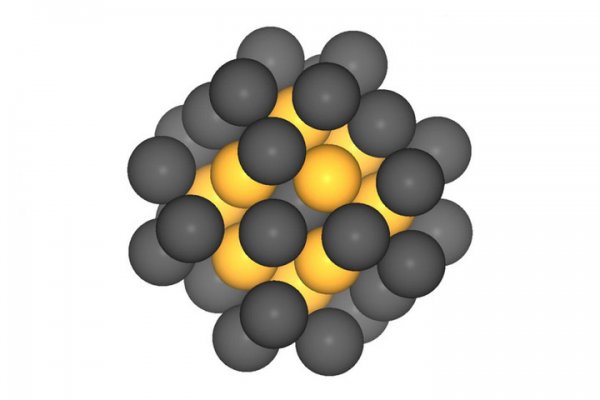
A platinum "egg" (particle) is only one nanometer in size